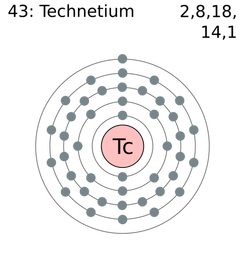
Technetium (tek-nee-shee-əm)Technetium is the 43rd element on the Periodic Table. It has 43 protons and 55 neutrons. It also has 43 electrons on 5 energy levels. It is a Transition metal in the 7th family and on the 5th period.
Technetium star
|
Extra: Fun Game LinkThe author of this site did not create this game. Have fun ;) http://www.purposegames.com/game/elements-technetium-game
|
Works Cited
"Major Groups of Elements: Transition Metals." The World Book Encyclopedia of Science. Chicago: World Book, 2000. 46. Print.
Parsons, Paul, and Gail Dixon. "Technetium." The Periodic Table: A Visual Guide to the Elements. New York: Quercus, 2014. 104-05. Print.
"Technetium." - Knowledge Encyclopedia. N.p., n.d. Web. 22 Oct. 2015.
"Technetium Element Facts." Chemicool. N.p., n.d. Web. 22 Oct. 2015.
"Technetium: Historical Information." Technetium»historical Information [WebElements Periodic Table]. N.p., n.d. Web. 22 Oct. 2015.
"Technetium." The New Book of Popular Science. Danbury, CT: Grolier, 2006. 45. Print.
"TECHNETIUM." Technetium, Chemical Element. N.p., n.d. Web. 22 Oct. 2015.
Parsons, Paul, and Gail Dixon. "Technetium." The Periodic Table: A Visual Guide to the Elements. New York: Quercus, 2014. 104-05. Print.
"Technetium." - Knowledge Encyclopedia. N.p., n.d. Web. 22 Oct. 2015.
"Technetium Element Facts." Chemicool. N.p., n.d. Web. 22 Oct. 2015.
"Technetium: Historical Information." Technetium»historical Information [WebElements Periodic Table]. N.p., n.d. Web. 22 Oct. 2015.
"Technetium." The New Book of Popular Science. Danbury, CT: Grolier, 2006. 45. Print.
"TECHNETIUM." Technetium, Chemical Element. N.p., n.d. Web. 22 Oct. 2015.