|
History and Discovery
In 1789, while analyzing the composition of the mineral, Jargon (ZrSiO4), Martin Heinrich Klaproth (who also discovered Uranium and Cerium) came across a new element. Zirconium. In 1824, it was isolated by a Swedish chemist, Jöns Jacob Berzelius. From the Persian word Zargun, which means gold-like, he named the new element Zirconium. Isolating Zirconium is a difficult process because it's found with deposits of hafnium, which shares similar chemical properties. Today, we use the Kroll Process to separate Hafnium and Zirconium. Both elements are found commonly in zircon (ZrSiO4) and baddeleyite (ZrO2). Abundance The abundance of an elements measures how common the element is compared to other elements in the environment. The abundance of Zirconium inside the Earth's crust is 65 parts per million by weight, 38 parts per million by moles. Also, in the solar system, 40 parts per billion by weight, 0.5 parts per billion by moles. You can gather quite a few things from this. Zirconium is more abundant in the Earth's crust than it is in the solar system. It is a pretty common element, an only costs $16 for a pound of bulk zirconium. Of course if wanted in pure form, it costs $157 per pound. Zirconium in Industry and Technology (Uses) Zircoium is very poor at taking in neutrons, so it is used in the outer layers of nuclear energy fuel rods, because it is important neutrons can pass through easily. It is also used in surgical equipment and steel alloys. Sometimes it is even used to make superconductive magnets. One of it's biggest uses, is fake diamonds. Cuba Zirconia (ZrO2) which is zirconium and two oxygens, is a low-cost substitute for a fake diamond. It Fact: There are over half-million linear feet of zirconium alloy tubing. Reactivity Zirconium is highly reactive. It can catch flame in the air, without a flame source if not fully dry or fully wet. It only takes a pinch of the powder, to light a bright white flame Sources Gray, Theodore W., and Nick Mann. The Elements: A Visual Exploration of Every Known Atom in the Universe. New York: Black Dog & Leventhal, 2009. Print. "The Element Zirconium." It's Elemental -. Jefferson Lab, n.d. Web. 29 Oct. 2015. "Zirconium Element Facts." Chemicool. N.p., n.d. Web. 29 Oct. 2015. |
Zirconium
Symbol: Zr
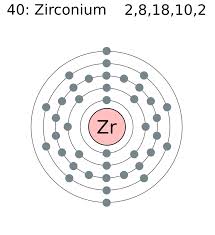
Atomic Number: 40
Atomic Mass: 91.22
Protons: 40
Electrons: 40
Neutrons: 51
Energy Levels: 5
Boiling Point: 4400 ºC, 4673 K
Malting Point: 1850 ºC, 2123 K
Density: 6.52 g/cm3
State at Room Temp: Solid