By Sofia Rhodes
Lithium Symbol. N.d. Pix For Lithium Symbol. Web. 22 Jan. 2015.
|
A trivia game for your entertainment :)
http://www.braingle.com/trivia/27071/lithium.html http://www.funtrivia.com/playquiz/quiz5539965a550.html |
Click on this to see Lithium in water!!!!
https://www.youtube.com/watch?v=vRKK6pliejs |
Discovery of Lithium
|
On the left: Jozé Bonifácio de Andralda e Silva
On the right: Johann August Arfvedson. |
Lithium was first "discovered" on the Swedish island of Utö by the Brazilian, Jozé Bonifácio de Andralda e Silva in the 1790s. Jozé didn't really discover Lithium he just found it and used it to make flames the color crimson red like they are today. Not until 1817 was it observed in mineral petalite form by Johann August Arfvedson. So it wasn't in its purest form but rather it was in an ore form. Johann later released it as an alkali metal and a lighter version than sodium. Not until later was it first isolated by William Thomas Brande and Sir Humphrey Davy through the electrolysis of lithium oxide (Li2O). They isolated it by passing an electric current through lithium oxide. Although it is an alkali metal it was discovered in mineral form unlike all the other alkali metals which were found in plant material. Some think it is where the greek root comes from which is Lithos which means "stone".
|
Reactiveness&Description
|
Lithium is the least reactive in the alkali metals group but still is highly reactive . It reacts the most with water because of all the moisture that is inside water. When lithium violently reacts with water it creates a compound of lithium hydroxide and liberating hydrogen. It can also react with the halogen family to create a lithium salt. The elements physical description has a sort of silvery luster from it as well as a hard looking outside. But looks perceive, and Lithium can be cut with a plastic knife right in half without even putting some elbow grease into it!
source: https://www.flickr.com/photos/37388341@N00/1490554529 |
Main uses in the Modern World
On the outside Lithium seems like one of the hardest metals, yet it can be cut by a knife easily. Because it is one of the lightest metals, when it is combined with Aluminum it can be used in airplane parts. Since it is so light airplanes want to use so there is little weight when flying. So when Lithium is combined with Aluminum which is a sturdy metal it creates the perfect combination for high-performance parts for an airplane. Also Lithium can be used as a bipolar medicine when combined with carbonate. Lithium acts on the central nervous system and the spinal cord which controls most of your behavior and actions. Doctors don't know how it, it exactly works but it is thought to help the nervous system communication in the brain regulating your mood and behavior. One of the most common uses of Lithium is in batteries. Lithium is ideal for use in battery applications as it has the highest electric output per unit weight of any battery material. Lithium ion batteries are slowly becoming one of the most popular batteries because they are some of the best rechargeable batteries. You can use them to power ipods, iPhones, computers etc.
Interesting Facts
1. Lithium is the only alkali metal that reacts with Nitrogen
2. Lithium is also believed to be one of the 3 elements that was mass produced during the first 3 minutes of the universe's creation (the big bang)
3. Lithium is coroding, causing skin burns as a result of the burning hydroxide produced in contact with moisture.
2. Lithium is also believed to be one of the 3 elements that was mass produced during the first 3 minutes of the universe's creation (the big bang)
3. Lithium is coroding, causing skin burns as a result of the burning hydroxide produced in contact with moisture.
Abundance of Lithium
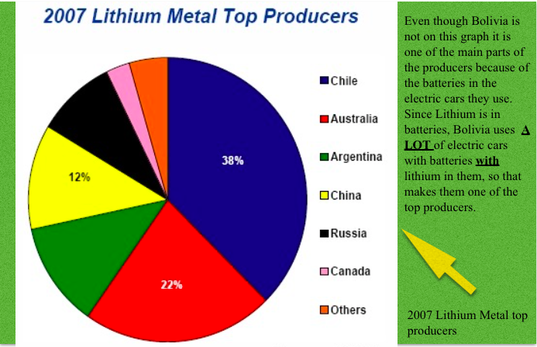
Lithium isn't the most popular element on the periodic table of elements unlike gold, silver or oxygen. Even though it's not the most popular it is abundant in the world. According to research in 2008 the Lithium count was about 10.6 million tons (that's a lot). Now in comparison to one of the popular elements like gold, gold only has 165,000 tons on the earth (just take a minute to soak in). The top 3 producers of Lithium are Australia, Chile and China. Now those countries are widespread all over the place so lithium isn't produced in just one certain area of the world. There's one country that isn't mentioned in the chart that also has a major impact on production of Lithium. Bolivia has a ton of electric cars that run on batteries. The batteries that they use have lithium in them. Since they have a ton of electric cars that means a ton of batteries which means a ton of Lithium. This metal is very abundant in sea water and is mined from brines and clay.
Sources
2 non-technology sources:
Johanson, Paula. Lithium. New York: Rosen Pub. Group, 2007. Print.
Gray, Theodore W., and Nick Mann. The Elements: A Visual Exploration of Every Known Atom in the Universe. New York: Black Dog & Leventhal, 2009. Print.
Electronic Sources:
"Lithium Element Facts." Chemicool. N.p., n.d. Web. 29 Oct. 2015.
Pappas, Stephanie. "Facts About Lithium." LiveScience. TechMedia Network, 23 Sept. 2015. Web. 29 Oct. 2015.
"Interesting Facts About Lithium: Surprising and Controversial, but ALL True." Bipolar Lives. N.p., n.d. Web. 29 Oct. 2015.
"Lithium Facts." Lithium Facts. N.p., n.d. Web. 29 Oct. 2015.
"Lithium." <i>Pictures, Stories, and Facts about the Element in the Periodic Table</i>. N.p., n.d. Web. 29 Oct. 2015.
"Lithium Abundance - World Lithium Reserve." <i>Lithium Abundance - World Lithium Reserve</i>. N.p., n.d. Web. 29 Oct. 2015.
"Lithium - Element Information, Properties and Uses | Periodic Table." Lithium - Element Information, Properties and Uses | Periodic Table. N.p., n.d. Web. 29 Oct. 2015.
"What Is It Used For? - Western Lithium Corporation." Western Lithium Corporation. N.p., n.d. Web. 29 Oct. 2015.
"Lithium Battery Shipments Face More Restrictions." <i>Industrial Distribution</i>. N.p., n.d. Web. 29 Oct. 2015.
"Lithium Treatment for Bipolar Disorder: Side Effects and More." WebMD. WebMD, n.d. Web. 29 Oct. 2015.
Johanson, Paula. Lithium. New York: Rosen Pub. Group, 2007. Print.
Gray, Theodore W., and Nick Mann. The Elements: A Visual Exploration of Every Known Atom in the Universe. New York: Black Dog & Leventhal, 2009. Print.
Electronic Sources:
"Lithium Element Facts." Chemicool. N.p., n.d. Web. 29 Oct. 2015.
Pappas, Stephanie. "Facts About Lithium." LiveScience. TechMedia Network, 23 Sept. 2015. Web. 29 Oct. 2015.
"Interesting Facts About Lithium: Surprising and Controversial, but ALL True." Bipolar Lives. N.p., n.d. Web. 29 Oct. 2015.
"Lithium Facts." Lithium Facts. N.p., n.d. Web. 29 Oct. 2015.
"Lithium." <i>Pictures, Stories, and Facts about the Element in the Periodic Table</i>. N.p., n.d. Web. 29 Oct. 2015.
"Lithium Abundance - World Lithium Reserve." <i>Lithium Abundance - World Lithium Reserve</i>. N.p., n.d. Web. 29 Oct. 2015.
"Lithium - Element Information, Properties and Uses | Periodic Table." Lithium - Element Information, Properties and Uses | Periodic Table. N.p., n.d. Web. 29 Oct. 2015.
"What Is It Used For? - Western Lithium Corporation." Western Lithium Corporation. N.p., n.d. Web. 29 Oct. 2015.
"Lithium Battery Shipments Face More Restrictions." <i>Industrial Distribution</i>. N.p., n.d. Web. 29 Oct. 2015.
"Lithium Treatment for Bipolar Disorder: Side Effects and More." WebMD. WebMD, n.d. Web. 29 Oct. 2015.