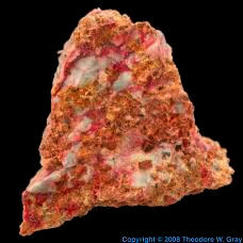
ARSENICP. PERKINS Arsenic is element number 33 on the periodic table. It has 23 isotopes. Only one of the isotopes are stable. It has 33 electrons, 33 protons, and 42 neutrons in it's most stable isotope. Arsenic has melting point of 1090 kelvin and a boiling point of 876 kelvin. The element's atomic mass is 74.922. Arsenic is one of only 2 elements to not melt at a normal pressure.
This element has been known all the way back since ancient Greece, with an unknown discoverer. It is thought that the element's name came from the Greek word arsenikos, meaning potent. Arsenic can be found in soil, rocks, air and water because of it's abundance in the earth's crust. It is contained in over 200 mineral compounds. It is also naturally found in almost every living thing, including humans. The main way it gets into the air is by volcanic eruptions. It mainly occurs naturally, though human activity can raise and lower levels in certain regions. In the universe as a whole, it is 12 parts per billion by weight. Because of arsenic's toxic properties, it is used in wood preservation and insecticides. Gallium arsenide, a compound of gallium and arsenic, is used as a semiconductor in LED's and laser diodes. Small amounts of arsenic are used in aluminum alloys for ammunition. Even though arsenic is a toxic element, it is an essential element to humans, with a level of 0.00001% needed for healthy growth and a healthy nervous system. In large quantities, arsenic can be dangerous to human health. Arsenic is not dangerous in small quantities, and it is contained in small amounts in your body, water, air, and even rice. When ingested in great amounts, it can cause skin, lung, bladder, and kidney cancer. Arsenic can also cause skin thickening and pigmentation. Arsenic causes damage to whole chromosomes, not just genes. |
VIDEOS
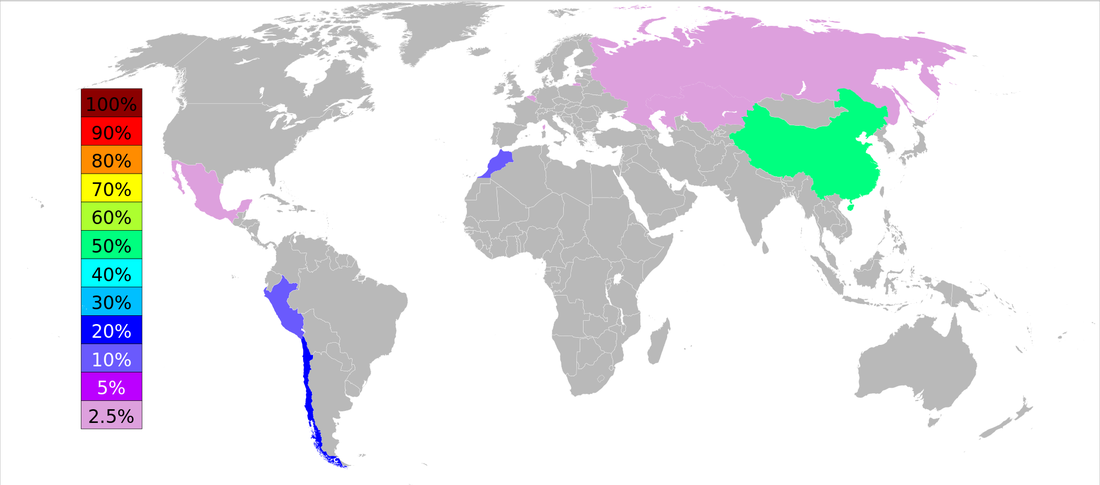
ARSENIC OUTPUT BY COUNTRY
ARSENIC OSHA SAFTEY SHEET
| 0038.pdf | |
| File Size: | 523 kb |
| File Type: | |
"s13.JPG." Periodic Table. N.p., n.d. Web. 20 Oct. 2015. <http://www.periodictable.com/Samples/
033.7/s13.JPG>.
"Arsenic." Chemicool. N.p., n.d. Web. 20 Oct. 2015. <http://www.chemicool.com/elements/
arsenic.html>.
"Arsenic." GreenFacts. N.p., n.d. Web. 20 Oct. 2015. <http://www.greenfacts.org/en/arsenic/l-2/
arsenic-3.htm#0>.
"World Arsenic Production 2006." Wikimedia. N.p., n.d. Web. 20 Oct. 2015.
<https://upload.wikimedia.org/wikipedia/commons/thumb/b/be/World_Arsenic_Production_2006.svg/
2000px-World_Arsenic_Production_2006.svg.png>.
033.7/s13.JPG>.
"Arsenic." Chemicool. N.p., n.d. Web. 20 Oct. 2015. <http://www.chemicool.com/elements/
arsenic.html>.
"Arsenic." GreenFacts. N.p., n.d. Web. 20 Oct. 2015. <http://www.greenfacts.org/en/arsenic/l-2/
arsenic-3.htm#0>.
"World Arsenic Production 2006." Wikimedia. N.p., n.d. Web. 20 Oct. 2015.
<https://upload.wikimedia.org/wikipedia/commons/thumb/b/be/World_Arsenic_Production_2006.svg/
2000px-World_Arsenic_Production_2006.svg.png>.